
Gas exchange: hypercapnia
In one study it was mentioned that hypercapnia is common during mechanical ventilation in patients with severe asthma, with an average arterial partial pressure of carbon dioxide (PaCO2) of 68 mm Hg and a pH of 7.18. Sometimes, PaO2 may exceed 100 mm Hg. Despite normal minute ventilation, hypercapnia is the result of a significant increase in physiological dead space caused by alveolar overdistension. The effect of increasing ventilation to reduce PaCO2 is therefore reduced by the further increase in dead space associated with progressive hyperinflation. Therefore, hypercapnia in the acute phase of asthma may not be truly 'permissible' as it may not be possible to normalise PaCO2 through ventilatory regulation.
Given the potential risk of malignant hyperinflation, a reasonable strategy may be to choose a ventilator setting that provides a safe level of dynamic hyperinflation (tidal volume of 7-9 mL/kg and respiratory rate of 10-14 breaths/min) and accept the resulting PaCO2 (see table below).
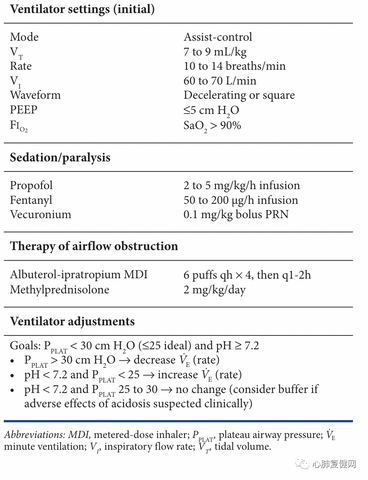
Fortunately, hypercapnia in the persistent state of asthma is usually well tolerated and serious adverse consequences are rare. The most serious consequence of hypercapnia is increased intracranial pressure in acute neurological patients. Although rare, cerebral oedema and subarachnoid haemorrhage due to hypercapnia have been reported in the context of persistent asthma. Hypercapnia is of greatest concern when there is cerebral hypoxia secondary to respiratory arrest prior to intubation. Unfortunately, unless the patient develops reversible bronchospasm, it may be difficult to achieve normal CO2 levels in fulminant asthma without extracorporeal CO2 clearance.
One way to manage acute respiratory acidosis in asthmatic patients is to receive elevated PaCO2 and buffering agents. Unfortunately, standard buffer therapy is relatively inefficient in acute respiratory acidosis. Even partial correction of severe respiratory acidosis usually requires at least a few hundred milligram equivalents of sodium bicarbonate in adults. However, in an animal study it was found that administration of sufficient sodium bicarbonate (14 mEq/kg) to maintain normal pH during induction of acute hypercapnia (PaCO2 = 80 mm Hg) prevented an increase in cerebral blood flow and intracranial pressure. If small amounts of bicarbonate are given slowly to raise the pH to 7.15 to 7.20, the effect on intracranial pressure is less certain. One problem with giving large amounts of bicarbonate is that once the airflow obstruction and hypercapnia have disappeared, the patient will be exposed to treatment-induced metabolic alkalosis.
In general, unless there is some convincing way to correct the underlying respiratory acidosis (e.g. arrhythmia, hyperkalaemia or other unexplained haemodynamic instability), pH correction should be abandoned and it may be reasonable to wait for PaCO2 to decrease as the airflow obstruction improves. Fortunately, many patients experience significant improvement in their hypercapnia within the first 12 hours of intubation. If bicarbonate therapy is given, ideally it should be administered by slow infusion rather than rapid push, as the latter may result in an acute increase in CO2 production and a dramatic drop in intracellular pH as a result of rapid diffusion of CO2 into the cells. An alternative to sodium bicarbonate is aminotriol (THAM), a buffering agent that does not produce CO2 during buffering. Although THAM may have some theoretical advantages over sodium bicarbonate in buffering respiratory acidosis, its use may still lead to problems with metabolic alkalosis.
See products here
https://www.smilecarehealth.com/collections/portable-oxygen-concentrator



